Study Statuses
Study Statuses
- Draft: Study has been created but has not yet been published and does not yet have Participants added. Any edits or changes should be made while in Draft status to ensure your Participants have the best experience.
- Active: Study has been published, is in progress, and may or may not have Participants added or contacted. Studies should have all setup steps filled in and reviewed to ensure accuracy before publishing to Active. Changing to “Active” opens your study for recruitment. Participants you've invited will be able to access any tests, surveys, screeners, or scheduling calendars in this study
- Closed: Study has been completed, no more changes are needed, and recruitment is closed. Participants won't be able to access any tests, surveys, screeners, or scheduling calendars in this study, and email sending is disabled.
- Paused: Study has been paused to stop Participant access to this study. Participants won't be able to access any tests, surveys, screeners, or scheduling calendars in this study, and email sending is disabled.
Update Study Statuses
Update Study Statuses easily from the main Studies page or a specific Study Overview page.
-
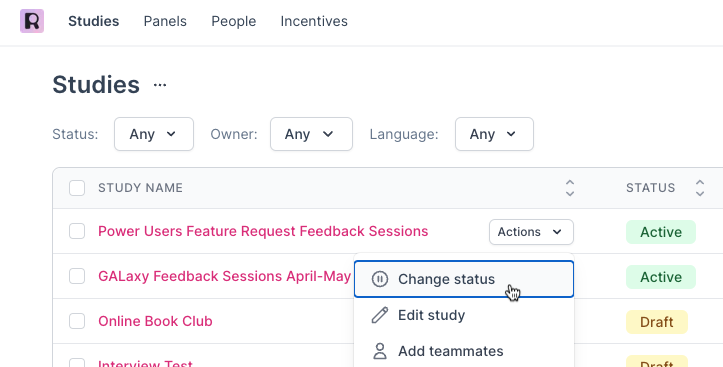
Navigate to the main Studies page in Rally and locate the relevant Study. Hover over the Study row and select 'Actions' and 'Change Status'. In the pop-up, select the new Study Status and review the changes that will be applied before selecting 'Update' to save the change.
-
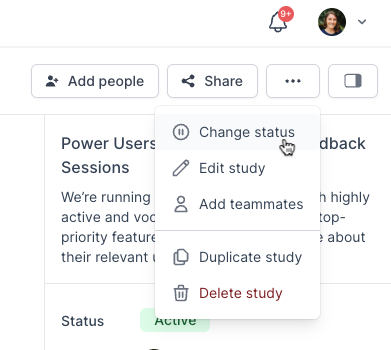
Navigate to the main Studies page and select the name of the relevant Study to open the Studies Overview page. In the top-right corner, select the three dots ... and 'Change Status'. In the pop-up, select the new Study Status and review the changes that will be applied before selecting 'Update' to save the change.
Archived Studies
Archive your Closed Studies to clean up your Studies page without deleting them. Participants will no longer be able to access any Study components. Participation history and data will be preserved.
-
Only Closed Studies can be Archived. To Archive a Closed Study, navigate to the Studies page and find the relevant Closed Study. Select 'Actions' and then 'Archive Study' and confirm in the pop-up that you would like to 'Archive Study'.
-
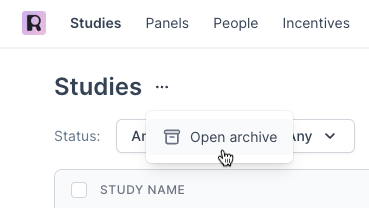
View Archived Studies by navigating to your Studies page and selecting the three dots ... next to the Studies title at the top.
-
Accidentally Archived Studies can be Unarchived and restored to your main Studies page as Closed Studies by navigating to Archived Studies using the steps above and locating the relevant Archived Study. Select 'Actions' and then 'Unarchive Study'. You can locate it again by navigating to your main Studies page and filtering for Studies with the "Closed" Status.
Participant Statuses
- Not Contacted: Participant has been added to a study but has not yet been contacted via email.
- Screener Sent: Screener email has been sent to the participant.
- Screener Completed: Participant has completed the screener.
- Scheduler Email Sent: Interview email has been sent to the participant.
- Interview Scheduled: Participant has scheduled an interview.
- Interview Completed: Participant has completed the interview.
- No Show: Participant agreed to the interview but did not attend the scheduled session.
- Canceled Interview: Interview was scheduled but either the participant or the host canceled it.
- Incentive Not Sent: Participant is still awaiting the incentive. Take action by sending an incentive email.
- Do Not Contact: Participant should not be contacted further.
Screener Statuses
- Screener Completed: Participant has submitted responses to the screener and may require review.
- Qualified: Participant is qualified to proceed with the study. Take action by sending them interview emails.
- Auto-Qualified: Participant is auto-qualified based on screener responses. Take action by sending them interview emails.
- Disqualified: Participant is not qualified to proceed with the study. Take action by sending a custom email thanking them for their responses.
Updated 3 days ago
